Stem cell therapy
Sunfield Clinic is a medical institution that provides regenerative medicine using stem cells.
by the Specially Certified Regenerative Medicine Committee, approved by the Ministry of Health, Labour and Welfare . Sunfield Clinic is a medical institution that has submitted an approved regenerative medicine plan to the Ministry of Health, Labour and Welfare and obtained a Type 2 regenerative medicine provision plan number.
(Classification of regenerative medicine, etc.: Type 2, Plan number PB3230222)
□ Regenerative medicine, etc. provided: “Autologous adipose-derived mesenchymal stem cell therapy for the relief of chronic pain”
(Classification of regenerative medicine, etc.: Type 2, Plan number PB3230228)
Stem-cell therapy
~The potential of mesenchymal stem cells~
These stem cells have long been known to exist in bone marrow. However, there is a limit to the number of stem cells that can be extracted from bone marrow, and extraction requires surgery that places a burden on the patient, such as general anesthesia. In 2000, Dr. Hedrick of UCLA (University of California, Los Angeles) discovered that abdominal subcutaneous fat contains large amounts of stem cells. A wide range of research has been conducted into the basic characteristics of adipose-derived mesenchymal stem cells. Adipose-derived mesenchymal stem cells are easy to extract, highly safe, and have a wide range of efficacy, making them garnering attention as key cells in regenerative medicine.
Furthermore, the Act on Ensuring the Safety of Regenerative Medicine, etc. (Act No. 85 of 2013) came into effect on November 25, 2014, establishing rules that medical institutions and cell processing product manufacturers must respect in order to ensure the safe progress of clinical research (such as clinical research using human stem cells) and the research, development, and practical application of high-quality regenerative medicine, etc., under the elective medical treatment system. This has created an environment in which regenerative medicine, etc., can be provided quickly and safely.
Sanfield Clinic has received regenerative medicine provision plan numbers from the Ministry of Health, Labor and Welfare, and offers the following treatments: “Autologous adipose-derived mesenchymal stem cell therapy for the prevention of the progression of arteriosclerosis” (Type 2, plan number PB3230222) and “Autologous adipose-derived mesenchymal stem cell therapy for the relief of chronic pain” (Type 2, plan number PB3230288).
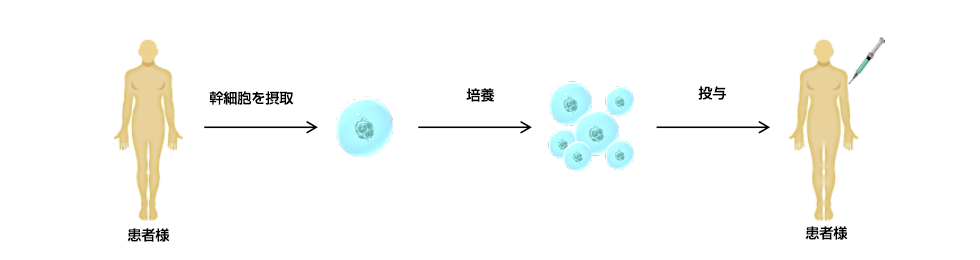
Flow of treatment
Prior explanation and consent
ㅤStem cell therapy can only be performed with the consent of both the patient and the doctor.
Pre-inspection
(Selection criteria and viral blood tests)fat harvesting
(Collection of subcutaneous adipose tissue)Local anesthesia is administered, and the amount collected is approximately 20 to 30 grams or more.
Stem cell culture/processing
ㅤStem cell cultivation is carried out under strict quality control at a contracted cell processing manufacturing facility.
Stem cell administration
(Administered by intravenous drip injection)is administered slowly over a period of about 40 minutes.
Follow-up examination
(Prognosis checkup)We will confirm the efficacy and safety of stem cell therapy for each patient.
cell culture

The adipose tissue collected from the patient is brought to the designated cell processing facility, where it is treated with enzymes and then the stem cells are isolated from the fat, cultured, and expanded. Cell culture is carried out under strict control.
To ensure a high level of safety, we conduct cell tests such as virus tests when the cells are brought in. Furthermore, to prevent mistakes in sample collection, cells are cultured under an operational management system that has introduced a strict quality control system, including history management for all processes.
Some products labeled as serum-free medium contain serum-derived substances. These carry the same risks as serum use and may not be suitable for clinical use from a safety perspective. The culture medium used at Sanfield Clinic is completely serum-free, even in its secondary raw materials.
・Standardize the culture process
・Does not depend on serum, allowing for the preparation of a variety of cells
Offered by Sunfield ClinicRegenerative medicine (stem cell therapy) provided by Sanfield Clinic
Autologous adipose-derived mesenchymal stem cell therapy for the prevention of arteriosclerosis progression
It has recently become clear that chronic inflammation occurs throughout the body as a disorder associated with aging. It is also known that arteriosclerosis, a typical vascular disorder, is triggered by chronic inflammation. In other words, aging, chronic inflammation, and arteriosclerosis are closely related events.
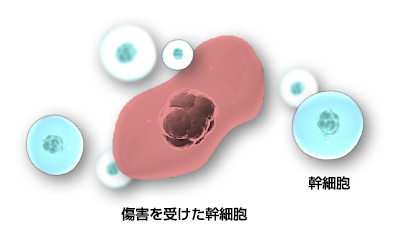
It is now known that arteriosclerosis is also caused by chronic inflammation, so it is thought that suppressing inflammation by administering mesenchymal stem cells will lead to the improvement or prevention of arteriosclerosis.
PB3230222
① Pulse wave velocity (PWV) ≧ 1400 (cm/s) or CAVI (Ankle Brachial Index) ≧ 8.0
② ABI test (Ankle Brachial Index) ≦ 0.9
③ Carotid ultrasound: Those with plaque (IMT ≧ 1.1) in the carotid artery (common carotid artery, internal carotid artery)
2) Those aged 18 or older
3) Those who are physically fit and in good health to withstand fat collection
4) Those who have normal capacity to consent, or those who can obtain consent from a legal representative
5) Those who have been given a consent form for this treatment, have received a sufficient explanation, and have obtained written consent of the patient’s own free will (if the patient does not have the capacity to consent, a legal representative can provide written consent)
6) Those whose doctor in charge has determined eligibility based on interviews, tests, etc.
There are other inclusion and exclusion criteria, so please contact the clinic for details.
*Follow-up examinations after stem cell administration (1, 3, 6, 12, 18, and 24 months after administration)
Treatment fee: 2,950,000 yen (excluding consumption tax)
bleeding from the wound, subcutaneous bleeding at the fat collection site, pain, infection, and exudate at the wound, side effects of anesthetics (nausea, vomiting, cold sweats, palpitations, etc.)
During stem cell administration:
anaphylactic reaction, shock, pulmonary embolism, difficulty breathing, decreased blood pressure, increased blood pressure, headache, cold sweat, nausea, vomiting, abdominal pain, nerve damage, numbness in the fingers and toes, pain at the puncture site, internal bleeding at the puncture site, erythema, rash, itching, edema, liver dysfunction, fatigue, fever, etc.
Autologous adipose-derived mesenchymal stem cell therapy for chronic pain relief

PB3230228
2) People who have not experienced satisfactory pain relief with other standard treatments for chronic pain, or who, despite fully understanding the standard treatments, do not wish to undergo treatment with the drugs used in standard treatments due to concerns about side effects,
etc. There are other inclusion and exclusion criteria, so please contact the clinic for details.
*Follow-up examinations after stem cell administration (1, 3, 6, and 12 months after administration)
Treatment fee: 2,950,000 yen (excluding consumption tax)
bleeding from the wound, subcutaneous bleeding at the fat collection site, pain, infection, and exudate at the wound, side effects of anesthetics (nausea, vomiting, cold sweats, palpitations, etc.)
During stem cell administration:
anaphylactic reaction, shock, pulmonary embolism, difficulty breathing, decreased blood pressure, increased blood pressure, headache, cold sweat, nausea, vomiting, abdominal pain, nerve damage, numbness in the fingers and toes, pain at the puncture site, internal bleeding at the puncture site, erythema, rash, itching, edema, liver dysfunction, fatigue, fever, etc.
We will provide you with detailed information and explanations about the treatment.
FAQ
What are stem cells?
A characteristic of stem cells is that they can produce cells that serve as the basis for other cells. When stem cells produce other cells, they divide into two. One of these divided cells remains as a stem cell, and the other cell becomes a cell that can transform into another cell. Stem cells create new cells in this way to replace damaged or old cells.
What is stem cell therapy?
Stem cells from the patient’s body are collected and mass-cultured at a CPC. After culturing, the stem cells may be administered into the bloodstream or transplanted directly into the affected area. For a detailed treatment procedure, please see Q.3 What is the process for stem cell therapy?
What is the process for stem cell therapy?
Stem cells from the patient’s body are collected and mass-cultured at a CPC. After culturing, the stem cells may be administered into the bloodstream or transplanted directly into the affected area. For a detailed treatment procedure, please see Q.3 What is the process for stem cell therapy?
Other menu
Culture supernatant treatment
Aging care
(infusion therapy/injections)